Publications
Dissecting Mechanisms Underlying Dendritic Cell Epigenetic Reprogramming
September 13, 2019
MSc. Thesis - Lund University
Svandís Þóra Sæmundsdóttir
Resources:
Abstract
Direct reprogramming refers to the ability of rewiring the transcriptional state of one lineage to another without passing through an intermediate pluripotent state. The ability to generate different cell types on demand with combinations of lineage-specific transcription factors (TFs) has opened new avenues for regenerative medicine and immunotherapy. It was recently established that fibroblasts transduced with PU.1, IRF8 and BATF3 results in cells with functional properties of dendritic cells (DCs). DCs are crucial cells in the immune system where they initiate adaptive immune responses, by incorporating and displaying antigens at the cell surface and present them to T-cells.
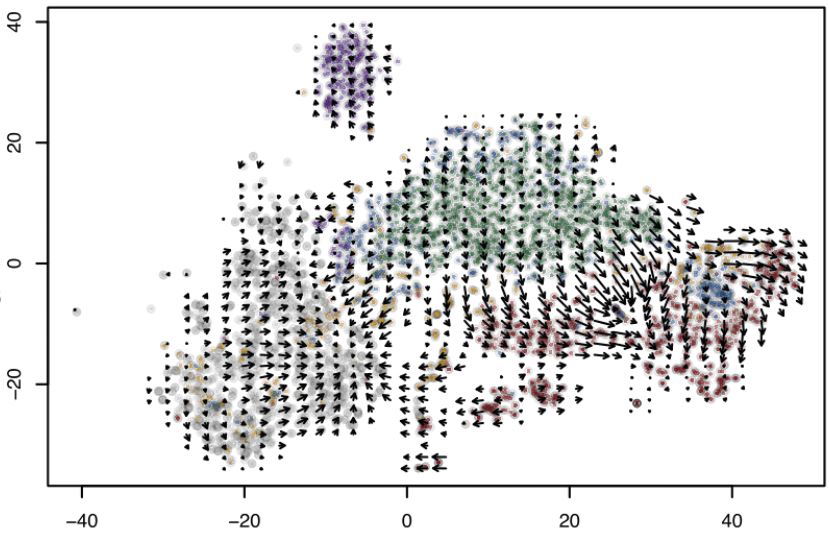
Here, I have done differential expression and pathway enrichment analysis on transcriptomic data obtained throughout reprogramming of mouse and human fibroblast into DCs. Differential expression analysis, correlation and PCA plot revealed reprogrammed mouse DCs share gene expression with “natural” DCs and t-SNE plot, heatmap and RNA velocity analysis showed reprogrammed human DCs favor a DC type 1-like fate. Pathway enrichment
analysis revealed lysosome, autophagy and inflammation as top pathways enriched throughout the reprogramming process. These pathways play an important part in DC maturation and I have identified candidate genes to address whether these pathways are important for the reprogramming process as well. Pathways that were seen decreased during reprogramming
process involved downregulation of cell division, reflecting the cell cycle status of mature DCs.
In this project, I provide evidence that the reprogramming process of fibroblasts into DCs is conserved between mouse and human based on gene expression analysis at the population and single cell levels. I further identified candidate genes in the lysosome, autophagy and inflammation pathways for future knockout/knockdown to verify their functional importance in DC reprogramming. Collectively this work increases our understanding on DC reprogramming mechanisms providing means to increase efficiency of the generation of human reprogrammed DCs for immunotherapy.