Publications
Cooperative Transcription Factor Induction Mediates Hemogenic Reprogramming

December 4, 2018 / Vol. 25, Issue 10
Cell Reports
Andreia M Gomes, Ilia Kurochkin, Betty Chang, Michael Daniel, Kenneth Law, Namita Satija, Alexander Lachmann, Zichen Wang, Lino Ferreira, Avi Ma'ayan, Benjamin K Chen, Dmitri Papatsenko, Ihor R Lemischka, Kateri A Moore, Carlos-Filipe Pereira
Related Data:
Population mRNA-seq of Reprogrammed Hemogenic Cells from HDFs Single Cell mRNA-seq of Umbilical Cord Blood and Reprogrammed Hemogenic Cells from HDFs ChIP-seq of GATA2, GFI1B and FOS on Reprogramming HDFsResources:
Highlights
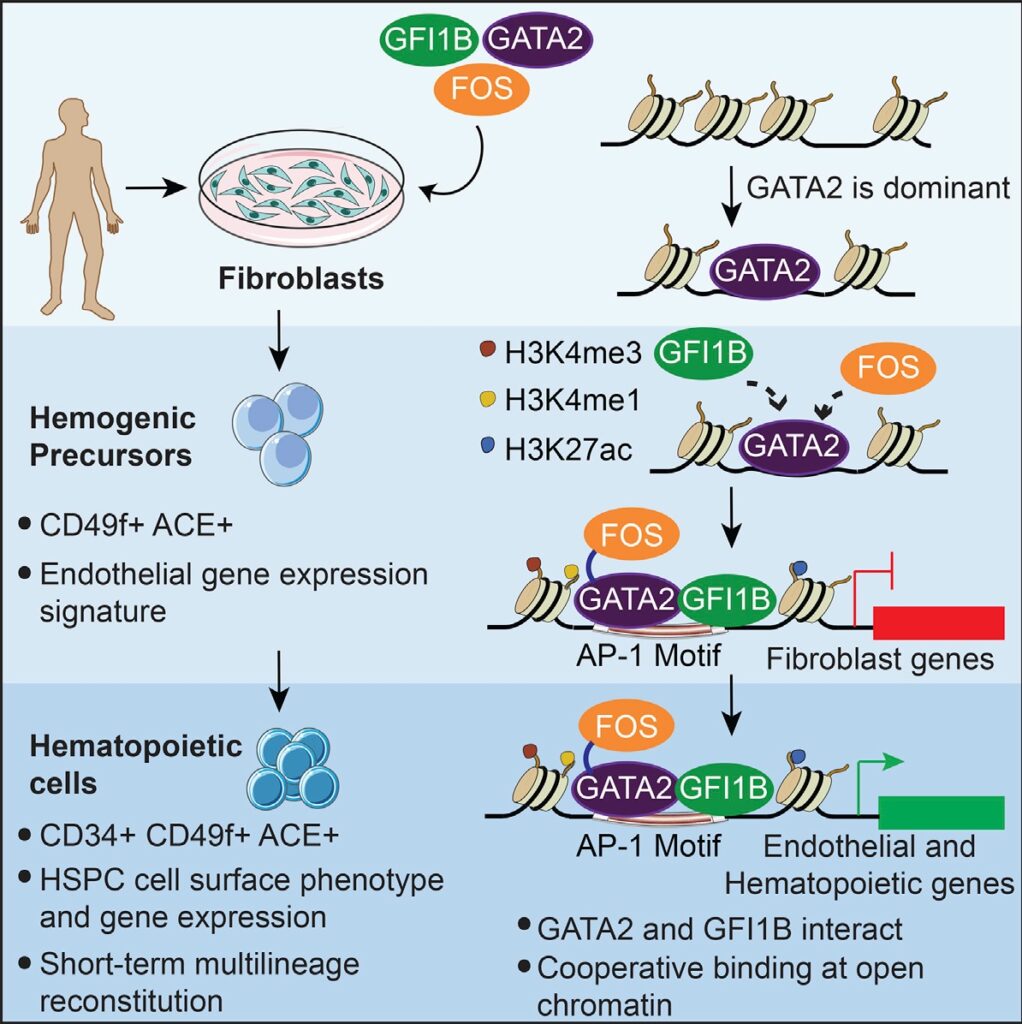
- GATA2, GFI1B, and FOS induce a hemogenic program in human fibroblasts
- Induced cells display dynamic endothelial-to-hematopoietic transition programs
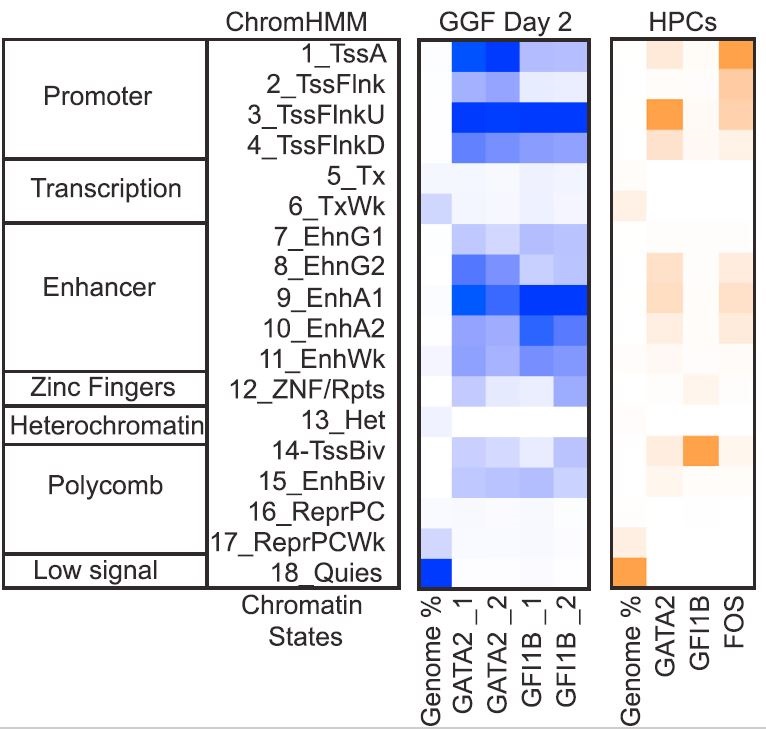
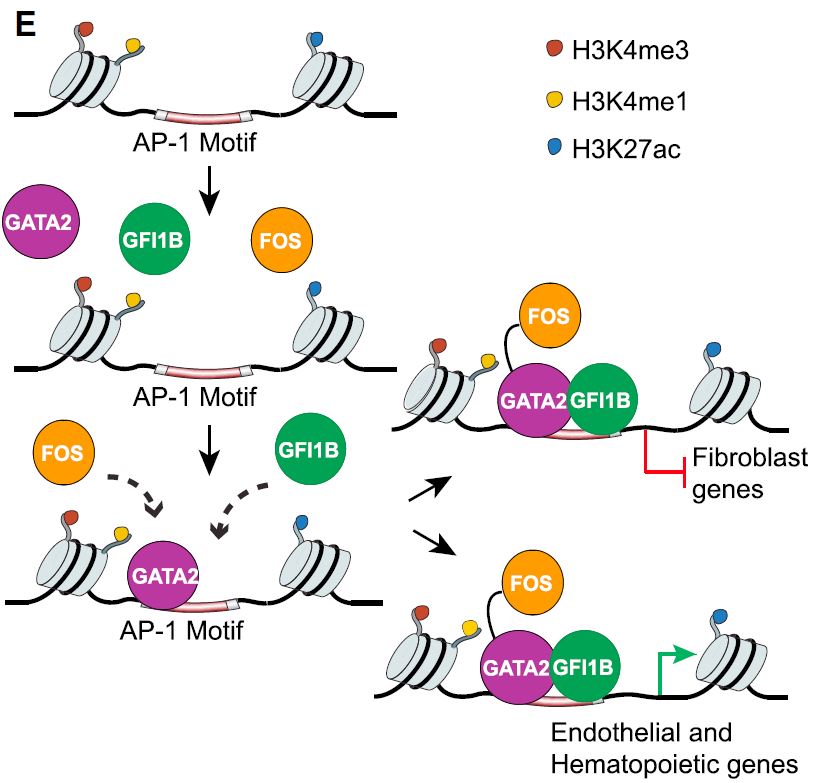
- GATA2 is the dominant transcription factor that initiates hemogenic reprogramming
- GATA2 and GFI1B interact and bind cooperatively at open chromatin
Abstract
During development, hematopoietic stem and progenitor cells (HSPCs) arise from specialized endothelial cells by a process termed endothelial-to-hematopoietic transition (EHT). The genetic program driving human HSPC emergence remains largely unknown. We previously reported that the generation of hemogenic precursor cells from mouse fibroblasts recapitulates developmental hematopoiesis. Here, we demonstrate that human fibroblasts can be reprogrammed into hemogenic cells by the same transcription factors. Induced cells display dynamic EHT transcriptional programs, generate hematopoietic progeny, possess HSPC cell surface phenotype, and repopulate immunodeficient mice for 3 months. Mechanistically, GATA2 and GFI1B interact and co-occupy a cohort of targets. This cooperative binding is reflected by engagement of open enhancers and promoters, initiating silencing of fibroblast genes and activating the hemogenic program. However, GATA2 displays dominant and independent targeting activity during the early phases of reprogramming. These findings shed light on the processes controlling human HSC specification and support generation of reprogrammed HSCs for clinical applications.