Research
Merging Stem Cells with Immunology by Cell Fate Reprogramming. Learn more about our research lines:
Modeling Hematopoietic Stem Cell Generation with Cell Reprogramming
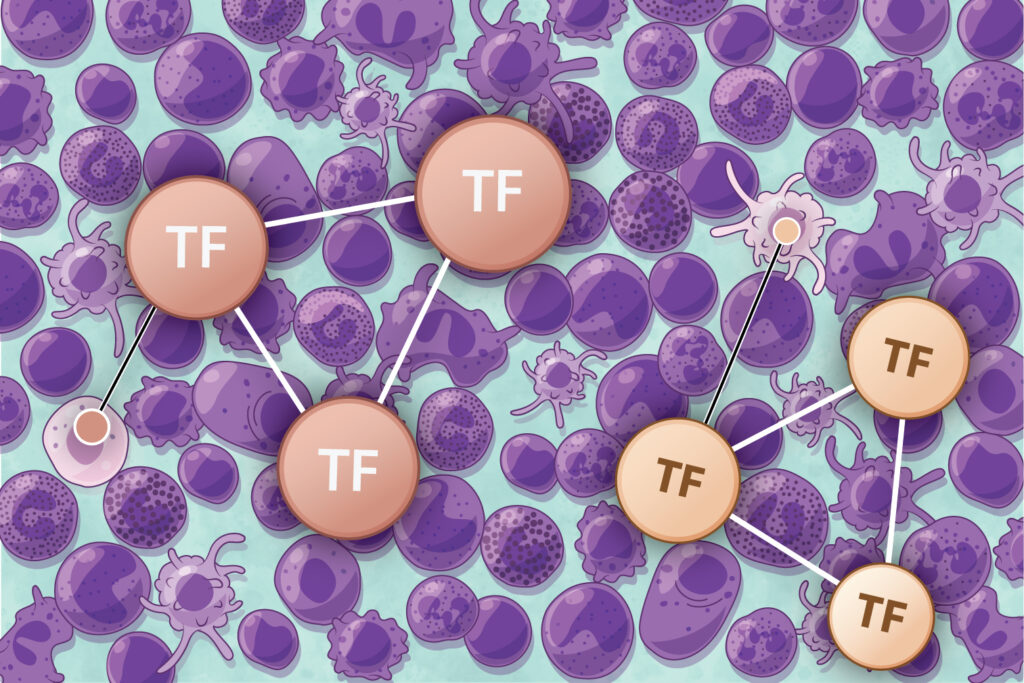
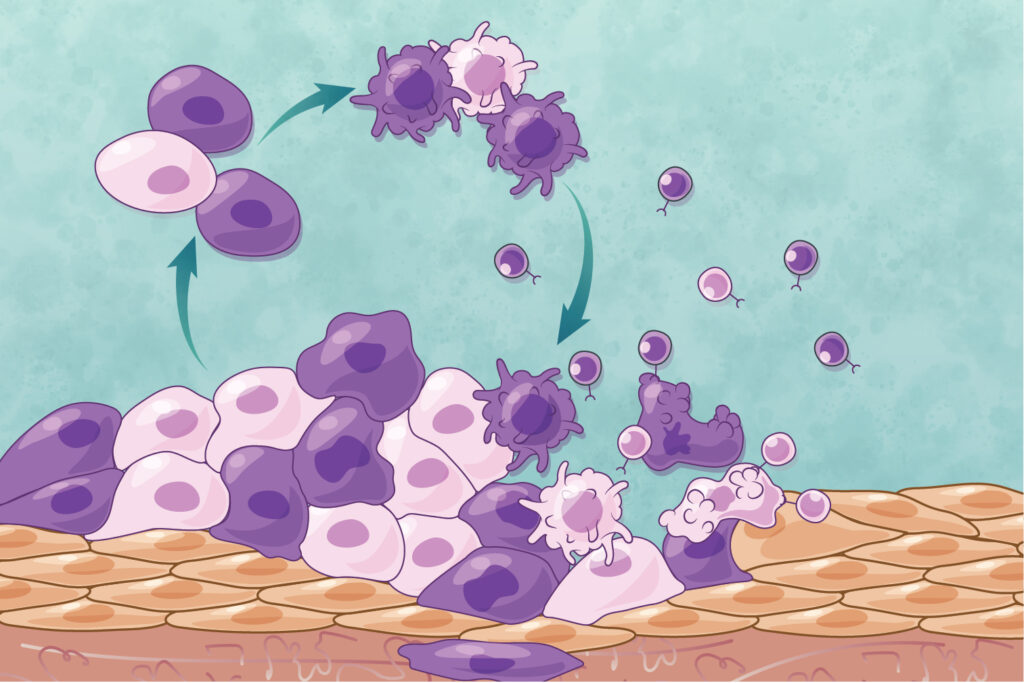
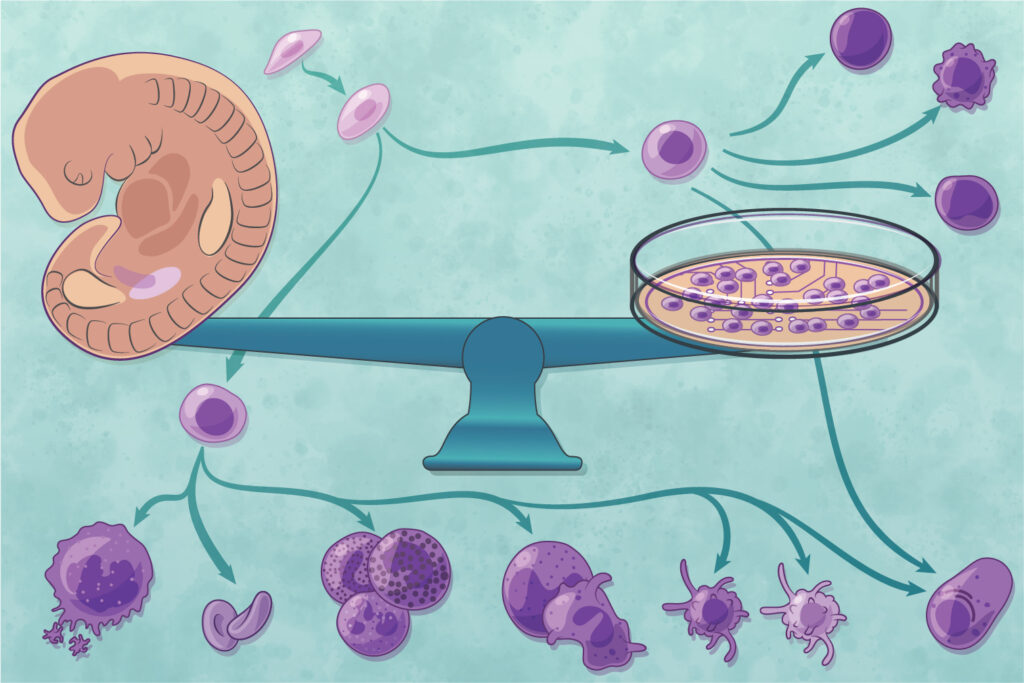
Hematopoietic Stem Cell (HSC) transplantation whether from bone marrow, mobilized peripheral blood or cord blood is the best example of regenerative cell therapy. HSCs have remarkable therapeutic applications in a broad range of diseases that affect the blood forming and immune system. Nevertheless, there remains a shortage of usable genetically-matched material for all needs. We employ an innovative approach to investigate the establishment of HSC identity: we screen for factors that re-create this unique cell state from fibroblasts. This approach leads to the identification of master transcription factors, cell surface phenotypes and gene expression signatures of induced cell fate. We then ask whether a similar logic applies to the specification of HSCs during mouse and human embryogenesis and use this information to uncover general principles of blood cell specification. We have demonstrated the direct reprogramming of mouse and human fibroblasts into clonogenic hematopoietic progenitors with the combination of transcription factors GATA2, GFI1B and FOS. We further established that cellular reprogramming approaches can inform the developmental specification of hematopoietic stem cells and shown that cooperative transcription factor binding mediates human hemogenic induction.