Publications
Using Direct Cell Reprogramming to Uncover Plasmacytoid Dendritic Cell Specification Programs and Function
June 17, 2021
MSc. Thesis - Lund University
Abigail Altman
Resources:
Abstract
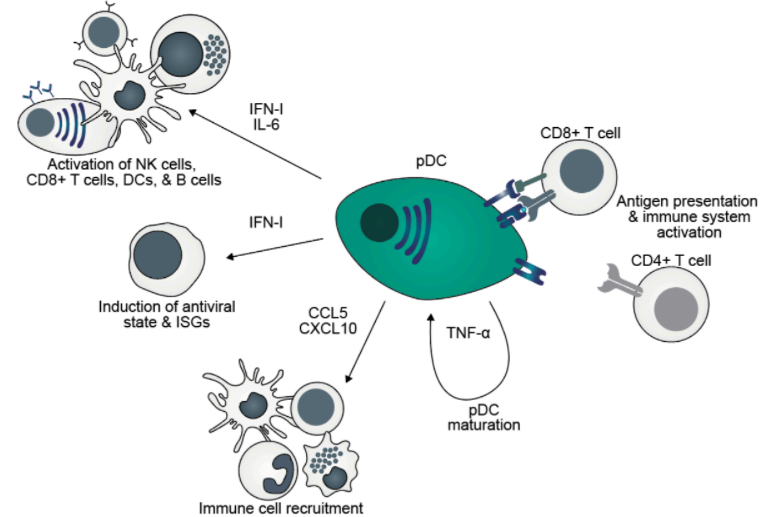
Direct cell reprogramming through enforced expression of transcription factors (TFs) converts somatic cells into functional, differentiated cells of other lineages without transiting through pluripotency. Recently, PU.1, IRF8, and BATF3 were identified as the TF network capable of reprogramming murine and human fibroblasts into conventional dendritic cells type 1 (cDC1s). Dendritic cells (DCs) are a remarkable heterogenous lineage bridging innate and adaptive immunity. In addition to antigen-presenting conventional DCs, plasmacytoid DCs (pDCs) represent a specific subset specialized at producing type I interferons (IFN-I) during the antiviral response. pDCs’ ontogeny and the TF networks required to specify pDC-fate and function remain unclear.
This thesis aims to define the TF codes required to induce functional pDCs from unrelated cell-types, utilizing cell fate reprogramming approaches. We have employed an IRF8- based screening approach, given its key role and high expression in pDCs. 36 candidate TFs were screened with IRF8 using mouse embryonic fibroblasts carrying a Clec9a- based reporter system. Overexpression of IRF8 and SPIB was sufficient to induce reporter activation. By performing a secondary screen, we identified IKZF2, when combined with IRF8 and SPIB, that in addition to maintaining Clec9a activation, increases hCD2-based reporter expression and kick-start expression of hematopoietic and pDC- specific surface markers. Thus these three TFs were identified as the optimal network to induce pDC-fate. Induced pDCs express the surface markers B220, CCR9, Ly6C, and antigen-presentation MHC-II molecules. Functionally, induced cells acquire the ability to secrete IFN-I and inflammatory cytokines including TNF-a, IL-6, CCL5, and CXCL10, in
response to TLR9 triggering.
This study identified a combination of three TFs to induce IFN-I-producing pDCs from a non-related cell type by direct cell reprogramming. This finding provides insight into the unique developmental program and functional properties of pDCs. In the future, these results open the possibility to explore induced pDCs to induce immune responses for immunotherapy.